Since Rembrandt,
etchers have used nitric acid and Dutch mordant to
etch copper. At the turn of the century ferric
chloride solution was introduced in gravure work,
principally because of its interaction with gum
bichromated gelatine. With the increased emphasis on
health and safety at work, we have had to reappraise
these and the other chemicals we use.
The salt, ferric
chloride, has none of the disadvantages associated
with the other two acid etching solutions: it does
not produce dangerous fumes, is odourless and, though
corrosive, is not absorbed through the skin.
Ferric chloride
solutions in water are strong acidic but in their
reaction with copper no significant fumes or gases
are produced. Simply, except in the presence of free
oxygen, copper will not react to any significant
extent with hydrochloric acid which is the acid
produced with ferric chloride solutions.
The chemistry
of the (etching) reaction
When ferric chloride
is dissolved in water the solution becomes strongly
acidic as a result of hydrolysis. The chemical
reactions, in words and formulae are:
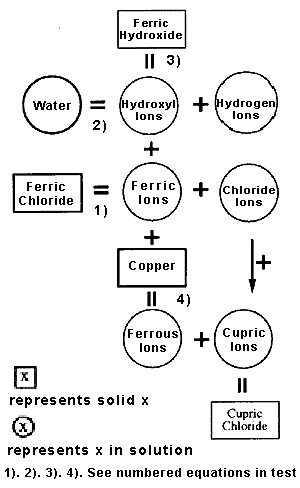
Schematic representation of the
chemical interactions occurring during etching copper
in ferric chloridesolution.
basically, ferric
chloride in water solution ionises to iron (ferric)
and chloride ions;
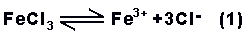
whilst the water
ionises to hydrogen and hydroxyl ions;
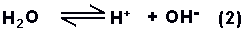
The ferric ions will
partially combine with the hydroxide ions to form
ferric hydroxide, a compound which is only slightly
soluble and precipitates from solution as a brown
solid.
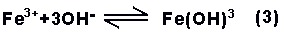
This precipitation can
be regarded as removing hydroxyl ions from the
solu-tion, leaving a relative excess of hydro-gen
ions and it is this excess that makes the solution
acidic. Because of a property of metals known as
electro-negativity, copper replace iron from the
solution and form a mixture of ferrous and cupric
ions. Effectively the copper dissolves without
producing any gas;
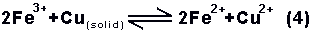
Ferrous ions become
increasingly stable as the solution becomes more
acidic and this helps the copper dis-solution to
proceed more easily whilst in basic (less acidic)
solutions the ten-dency is for ferrous ions to
convert back to ferric. As more copper dissolves and
the solubility limit is exceeded, cupric chloride
precipitates from the solution, as anything from a
green to a blue solid.
Strengths and
mixing
Ferric chloride can be
obtained either as a solid (not recommended as it can
give off highly toxic hydrogen chloride fumes when
mixed with water) or in liquid form when it is
usually supplied in a strength of 45º Baume. This
mea-surement equates to a specific gravity of
approximately 1.43 or a weight to volume ratio of
39-41%. Purveyors of ferric chloride can use any of
these measure-ments and often profess complete
igno-rance of any form of the others!
To form the basic
stock etching solu-tion, water is added in the ratio
2 parts water to 1 part ferric chloride solution
resulting in a solution strength of 42º Baume.
Preparing the
solution
At this stage the
strongly acidic ferric chloride requires further
treatment to remove free acid and to 'condition the
solution'. Either small quantities of copper may be
added and allowed to dissolve or (a more effective
way) take 10 cc of the stock solution and add 10 cc
of 9% household ammonia solution. This results in a
sludge of ferric hydrox-ide which is allowed to
settle before pouring off the liquid and adding the
precipitate to 1 litre of the stock solu-tion. Once
an etching cycle using ferric chloride has been
initiated the third method is probably easier. Here
spent ferric chloride liquor (containing ferric
hydroxide) is added to the new stock 42º Baume
solution, in the proportion 1 part spent liquor to 10
parts 42º Baume solution.
Colour in use
The optimum etching
conditions usu-ally occur after some etching has been
done (this reflecting an 'incubation' period for the
chemical reaction to 'settle down') but as etching
proceeds the solution becomes less effective. The
progress of this exhaustion can be oh served by the
colour changes - from an initial red brown (ferric
hydroxide) through a turbid (muddy) brown (ferric and
ferrous hydroxide) when the solution is working at
its most effi-cient, and finally to a dark green
solu-tion and black precipitate (cupric salts in
solution and precipitated with the hydroxides). At
this stage the solution is exhausted and requires
disposal. This can be effected by the slow addition
of either calcium carbonate (whiting) or sodium
carbonate (washing soda crystals) - until
effervescence ceases. At this point the solution is
neutralised and may be disposed of safely.
The myth
upside down
The normal
recommendation is to bite copper plates in ferric
chloride in-verted or on edge to avoid precipitated
ferric hydroxide clogging the etched line, thus
halting etching. However, there is little need for
this if the plate is bitten face up' and removed from
the solution every 20 min then washed in cold running
water to remove the pre-cipitate before continuing to
bite. This procedure does not present the hazard of
other etchants and has the advantage of checking
progress. Extremely deep bites or plates for relief
etching are still bitten upside down. On completion
of any biting, or if the plate is to stand between
bites for any length of time, it should always be
cleaned thoroughly with running water, since residual
ferric chloride in bitten lines will continue to
react.
Ease of use
To summarise, ferric
chloride is a chemical and should be handled with
respect. However, it does not present the level of
hazard associated with nitric acid and Dutch mordant;
indeed the only precautions necessary are to use
goggles and gloves, keep the solution in a plastic
bath, and wash off any liquid on the skin with water.
Extraction and fume cupboards are not necessary.
A 42º Baume solution
at its most ef-fective is very good for a deep line
bite. When diluted to approximately 32º Baume by the
addition of an equal part of water, it becomes very
effective for soft ground and delicate work without a
noticeable loss of biting time, and re-duces foul
biting. Due to the low depth of bite necessary in
aquatint, the strength of solution does not greatly
affect time taken to create a black, therefore
strength of solution tends to be a personal taste.
The minus side is the length of time taken to bite a
plate: half an hour in stock solution for a black
aquatint, and an hour for an aver-age etched line.
Very deeply bitten lines may take several hours.
Notes
1 To speed the
process, some sources recommend agitation of the
solution by bubbling air through an upright tank.
Whilst this undoubtedly works there is a risk of
hydrogen production by reac-tion with hydrochloric
acid. Therefore either extraction or a constant and
ade-quate flow of air is required to prevent hydrogen
build up.
2 It is preferable to
use industrial grade 45º Baume ferric for both cost
and speed (obtainable in the UK from Ellis and
Everard) these less refined solutions often bite
faster and it is the safest way t&) buy the salt.
Merck chemi-cals sell small quantities for laboratory
use but this is the Analar pure grade at a 60% weight
per volume ratio. This needs greater dilution to 42º
Baume and tends to be a slower acting form of the
salt.
Further
reading
N.I. Sax. Dangerous properties of
industrial materials, 1984. ISBN
0442-27373-8.
R.J. Lewis. The rapid guide to hazardous
chemicals in the workplace. ISBN 0442-
01759-6.
Contact: Steve
Hoskins, University of the West of England, Clanage
Road, Bower Ashton, Bristol BS3 2JT, UK
Email S2-Hoski@UWE.AC.UK.
 The information resource for printmakers
The information resource for printmakers
 The information resource for printmakers
The information resource for printmakers